
The Wild Growth of Plant-Based Meat Substitutes
March 18, 2020
Celebrating 60 Years, Building The ACE Way
July 6, 2020
As the world faces a shortage of pharmaceuticals and medical supplies, the U.S. Government is calling for increased production of these essential goods within the United States. In recent years, much of the pharmaceutical production has been moved overseas. More than 90% of the U.S.’s antibiotics, vitamin C, ibuprofen and hydrocortisone and 70% of acetaminophen are produced by Chinese pharmaceutical companies.1 To combat current and potential shortages, President Trump has called for companies to shift production back to America, invoking the Defense Procurement Act, which can be applied to address pharmaceutical and medical supplies shortages.2 Factory closings, transportation restrictions, and a large increase in demand have already put a strain on the supply of pharmaceuticals and medical supplies, such as protective masks, coming from China, further illustrating the need for increased U.S. production. The U.S. government continues to explore additional incentives to encourage more private companies to increase or begin production, including Buy American laws to help not only the short-term supply shortage, but also to decrease the long-term reliance on essential goods manufactured in foreign countries.1
Pharmaceuticals are not the only essential goods needed to fight COVID-19 that are in short supply. Medical supplies such as nylon-plastic swabs, plastic vials, and chemical reagents used in tests, surgical gloves, surgical masks, ventilators, and even hand-sanitizers and personal cleaning products are in short-supply.2 Some of these products are receiving increased production from unlikely places. American breweries and distilleries have started to make and distribute hand sanitizer to help fight the current shortage.3 Medical device manufacturers are ramping up their production to combat the pandemic as well. Fellow Chicago-area based company Abbott Labs has developed and quickly scaled production on their molecular point-of-care tests to detect novel coronavirus.4 Within two weeks of FDA approval, Abbott Labs tests have already been implemented in some of the most test needy cities, starting with Detroit earlier this month.5

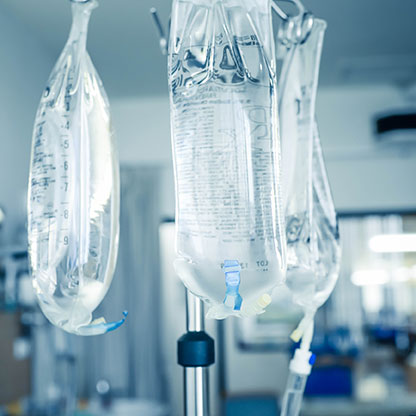
The path to a more sustainable American supply of essential medical supplies and pharmaceuticals already has a blueprint in place. Global pandemics are not the only cause that can lead to supply shortages of essential goods. War and natural disasters, such as 2017’s Hurricane Maria, can cause massive amounts of stress on the supply chain. The hurricane’s destruction to Puerto Rico’s infrastructure significantly hampered IV fluid bag production by market leader Baxter.6 With 8% of the medicine Americans take being supplied from Puerto Rico7, the hurricane had an extremely long-lasting impact on the United States’ access to medical supplies. To combat the short-term shortage, restrictions were lifted, allowing for IV bags to be temporarily imported from Baxter’s other facilities in Ireland, Australia, Canada, Mexico, Brazil, and England.8
With IV bag shortages resulting from the 2017 hurricane lasting through 2019, shifts in production were necessary to alleviate the long-term supply shortage. Baxter moved to diversifying its production locations for IV bags, coordinating with the FDA to get permanent approval from their other facilities. The FDA has currently approved some products to be imported from Baxter’s facilities in Ireland, Spain, and Mexico. Additionally, other companies have stepped up to meet the demand, taking heed of the dangers of relying on one location to produce all of these essential goods. B. Braun recently invested $1 billion into IV fluid manufacturing, updating their facility in Irvine, CA and strategically building a new facility in Daytona Beach, FL to mitigate risks from natural disasters.

At ACE METAL CRAFTS COMPANY, we recognize the importance of diversifying production locations for pharmaceuticals and essential medical supplies. If a natural disaster or pandemic knocks out a key cog in the supply chain, production of these life-saving goods stops. When multiple locations are producing these essential goods, one location can help pick up the slack if another area is hit by a disaster. With the push for increased pharmaceutical and medical supplies in the U.S., more capital will be spent on the production of these goods. However, these recent stresses to necessary medical supplies production have created a need to look differently at ROI, placing more of an emphasis on risk-mitigation. As pharmaceutical production companies shift to “just in time” logistics, ACE will help by suppling high-quality, stainless-steel components helping OEMs ramp up production, stocking these companies with the necessary equipment they need to produce the volume required or to get multiple facility locations fully equipped for production.
[1] Ana Swanson Coronavirus Spurs U.S. Efforts to End China’s Chokehold on Drugs
[2] Nishan Degnarain Coronavirus Crisis Shines Light On Sustainability In Global Pharma And Medical Supply Chain
[3] Michael Levenson Anheuser-Busch and Distilleries Race to Make Hand Sanitizer Amid Coronavirus Pandemic
[4] Stephanie Goldberg Abbott ramps up production of new COVID-19 test
[5] Syma Chowdhry Detroit will be first city to use new 15-minute COVID-19 tests today
[6] David Lim B. Braun invests $1B in IV fluid manufacturing to alleviate shortages
[7] Adam Aton Hurricane Maria Takes a Toll on Global Medical Supplies
[8] Baxter A Global Response to Hurricane Maria
Stock images © Getty Images





